Advanced Multiphoton Microscopy In Vivo
Summary
We aim to improve the imaging speed, resolution, penetration depth, and functionality of fluorescence microscopes using a variety of novel engineering techniques. These advanced techniques include, but are not limited to, multiphoton microscopy (MPM), fluorescence lifetime imaging microscopy (FLIM), super-resolution microscopy, adaptive optics, and deep learning methods. We design and build novel microscopes and collaborate with biologists to work on important problems in life sciences.
Super-Sensitivity MPM-FLIM
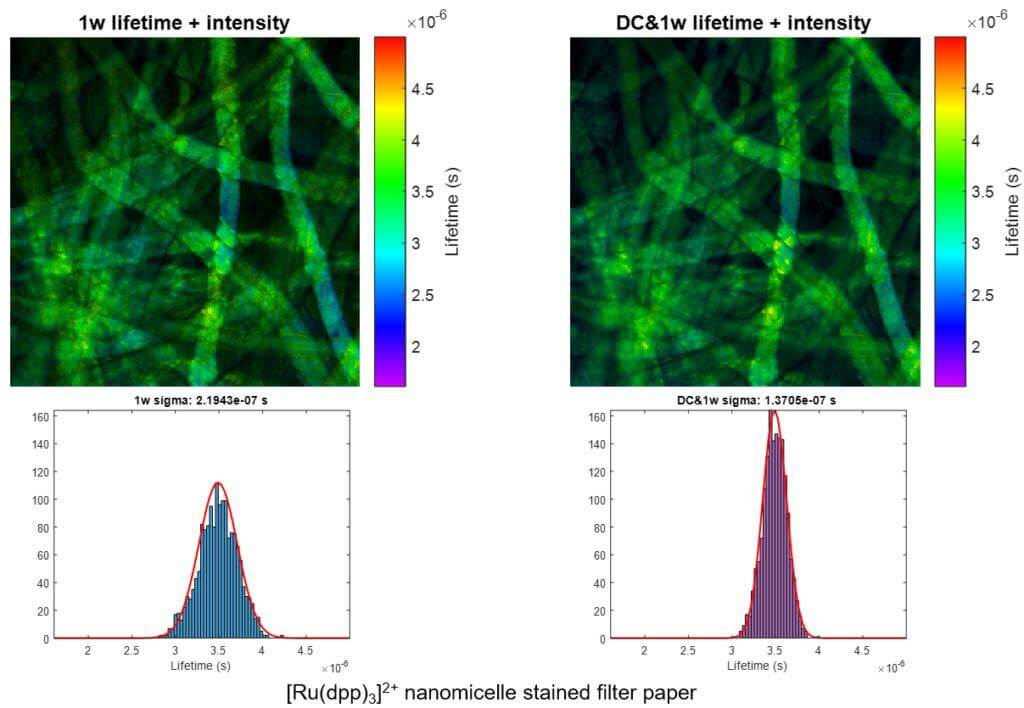
We present a series of experiments that demonstrate a super-sensitive chemical imaging technique based on multiphoton frequency-domain fluorescence lifetime imaging microscopy (MPM-FD-FLIM) that shows a 2x improvement in imaging speed compared to the theoretical limit of conventional MPM-FD-FLIM. Additionally, this technique produces unprecedented sensitivity over a large range of fluorescence lifetimes. These results are achieved through simple modifications to data analysis in a conventional MPM-FD-FLIM microscope and are based on an analytical model describing the signal-to-noise ratio (SNR) of a MPM-FD-FLIM system [J. Opt. Soc. Am. A 33, B1 (2016)].
Related Publications:
- Yide Zhang, Aamir A. Khan, Genevieve D. Vigil, and Scott S. Howard, “Investigation of signal-to-noise ratio in frequency-domain multiphoton fluorescence lifetime imaging microscopy”, Journal of the Optical Society of America A, vol. 33, no. 7, pp. B1-B11, July 2016, doi: 10.1364/JOSAA.33.0000B1.
- Yide Zhang, Aamir A. Khan, Genevieve D. Vigil, and Scott S. Howard, “Super-sensitivity multiphoton frequency-domain fluorescence lifetime imaging microscopy”, Optics Express, vol. 24, no. 18, pp. 20862-20867, Sept. 2016, doi: 10.1364/OE.24.020862.
- Yide Zhang, Genevieve D. Vigil, Aamir A. Khan, and Scott S. Howard, “Doubling the sensitivity of multiphoton frequency-domain fluorescence lifetime images” CLEO: Science and Innovations 2017, San Jose, California USA, May 2017, doi: 10.1364/CLEO_SI.2017.SM3C.6.
Saturation-Compensated FLIM
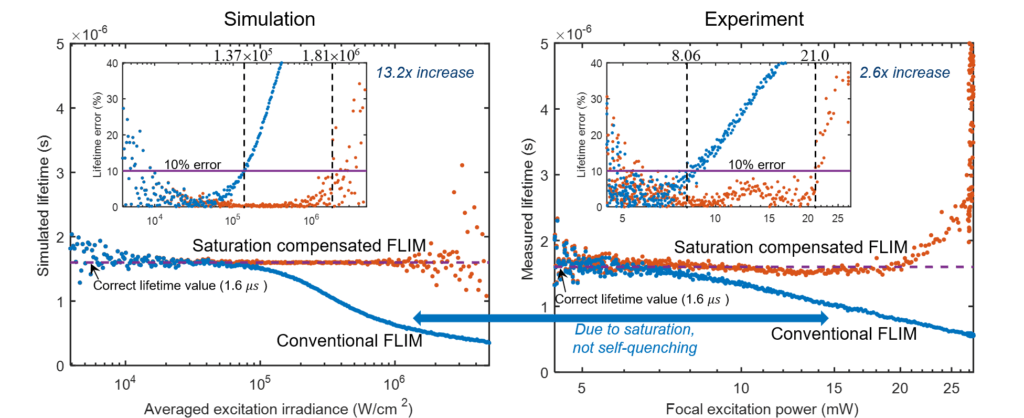
Fluorophore saturation is the key factor limiting the speed and excitation range of fluorescence lifetime imaging microscopy (FLIM). For example, fluorophore saturation causes incorrect lifetime measurements when using conventional frequency-domain FLIM at high excitation powers. We present an analytical theoretical description of this error and present a method for compensating for this error in order to extract correct lifetime measurements in the limit of fluorophore saturation. We perform a series of simulations and experiments to validate our methods. The simulations and experiments show a 13.2× and a 2.6 × increase in excitation range, respectively. The presented method is based on algorithms that can be easily applied to existing FLIM setups.
Related Publications:
- Yide Zhang, Genevieve D. Vigil, Lina Cao, Aamir A. Khan, David Benirschke, Tahsin Ahmed, Patrick Fay, and Scott S. Howard, “Saturation-compensated measurements for fluorescence lifetime imaging microscopy”, Optics Letters, vol. 42, no. 1, pp. 155-158, Jan. 2017, doi: 10.1364/OL.42.000155.
Super-Resolution Microscopy: Stepwise Optical Saturation (SOS)
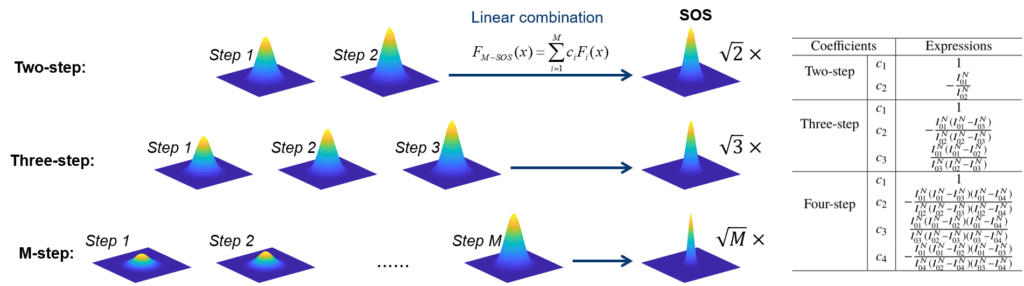
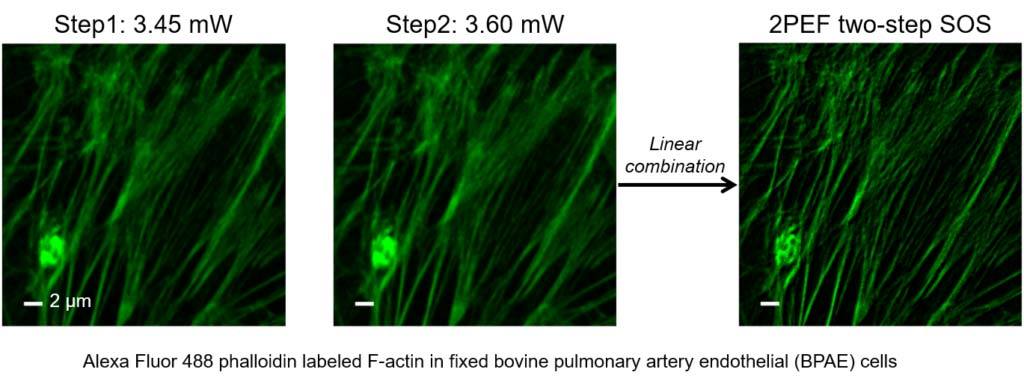
Super-resolution fluorescence microscopy is an important tool in biomedical research for its ability to discern features smaller than the diffraction limit. However, due to its difficult implementation and high cost, the super-resolution microscopy is not feasible in many applications. We propose and demonstrate a saturation-based super-resolution fluorescence microscopy technique that can be easily implemented and requires neither additional hardware nor complex post-processing. The method is based on the principle of stepwise optical saturation (SOS), where M steps of raw fluorescence images are linearly combined to generate an image with a √ M-fold increase in resolution compared with conventional diffraction-limited images. For example, linearly combining (scaling and subtracting) two images obtained at regular powers extends the resolution by a factor of 1.4 beyond the diffraction limit. The resolution improvement in SOS microscopy is theoretically infinite but practically is limited by the signal-to-noise ratio. We perform simulations and experimentally demonstrate super-resolution microscopy with both one-photon (confocal) and multiphoton excitation fluorescence. We show that with the multiphoton modality, the SOS microscopy can provide super-resolution imaging deep in scattering samples.
Related Publications:
- Yide Zhang, Prakash D. Nallathamby, Genevieve D. Vigil, Aamir A. Khan, Devon E. Mason, Joel D. Boerckel, Ryan K. Roeder, and Scott S. Howard, “Super-resolution fluorescence microscopy by stepwise optical saturation”, Biomedical Optics Express, vol. 9, no. 4, pp. 1613-1629, Apr. 2018, doi: 10.1364/BOE.9.001613.
- Yide Zhang, David Benirschke, and Scott S. Howard, “Stepwise optical saturation microscopy: obtaining super-resolution images with conventional fluorescence microscopes”, Biomedical Optics 2018, Hollywood, Florida USA, Apr. 2018, doi: 10.1364/TRANSLATIONAL.2018.JTh3A.27.
Super-Resolution FLIM: Generalized Stepwise Optical Saturation (GSOS)
We present a novel super-resolution fluorescence lifetime microscopy technique called generalized stepwise optical saturation (GSOS) that generalizes and extends the concept of the recently demonstrated stepwise optical saturation (SOS) super-resolution microscopy [Biomed. Opt. Express 9, 1613 (2018)]. The theoretical basis of GSOS is developed based on exploring the dynamics of a two-level fluorophore model and using perturbation theory. We show that although both SOS and GSOS utilize the linear combination of M raw images to increase the imaging resolution by a factor of √ M, SOS is a special and the simplest case of GSOS. The super-resolution capability is demonstrated with theoretical analysis and numerical simulations for GSOS with sinusoidal and pulse-train modulations. Using GSOS with pulse-train modulation, super-resolution and fluorescence lifetime imaging microscopy (FLIM) images can be obtained simultaneously. The super-resolution FLIM capability is experimentally demonstrated with a cell sample on a custom-built two-photon frequency-domain (FD) FLIM system based on radio frequency analog signal processing. To our knowledge, this is the first implementation of super-resolution imaging in FD-FLIM.
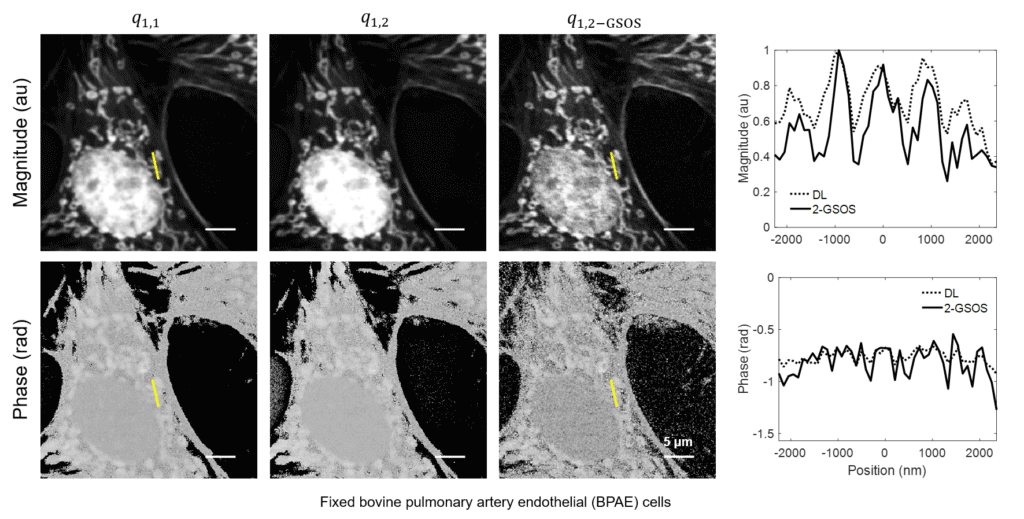
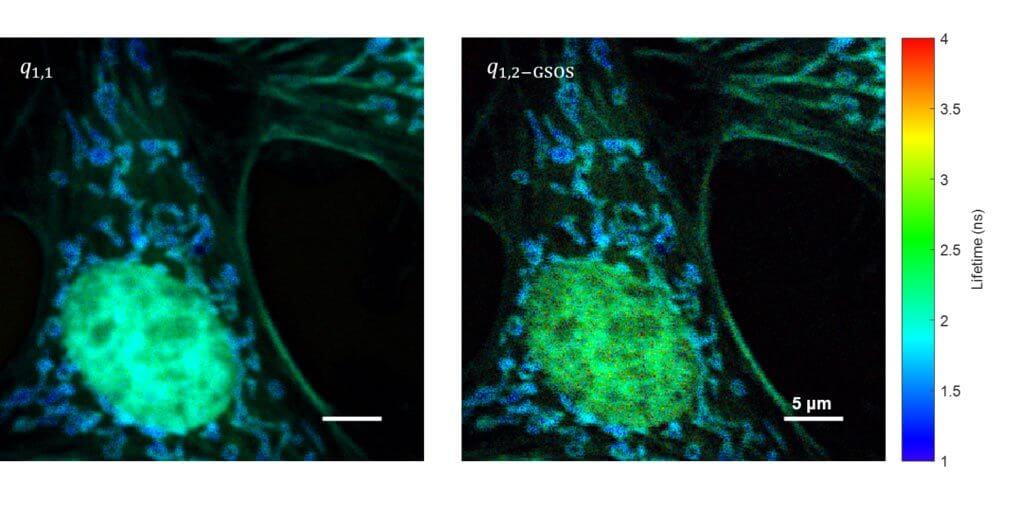
Related Publications:
- Yide Zhang, David Benirschke, Ola Abdalsalam, and Scott S. Howard, “Generalized stepwise optical saturation enables super-resolution fluorescence lifetime imaging microscopy”, Biomedical Optics Express, vol. 9, no. 9, pp. 4077-4093, Sept. 2018, doi: 10.1364/BOE.9.004077.
- Yide Zhang, David Benirschke, Ola Abdalsalam, and Scott S. Howard, “Super-resolution multiphoton frequency-domain fluorescence lifetime imaging microscopy by generalized stepwise optical saturation (GSOS)”, SPIE Photonics West 2019, San Francisco, California USA, Feb. 2019.
Phase-Multiplexing FLIM and Phasor Plots
We propose and demonstrate a novel multiphoton frequency-domain fluorescence lifetime imaging microscopy (MPM-FD-FLIM) system that is able to generate 3D lifetime images in deep scattering tissues. The imaging speed of FD-FLIM is improved using phase multiplexing, where the fluorescence signal is split and mixed with the reference signal from the laser in a multiplexing manner. The system allows for easy generation of phasor plots, which not only address multi-exponential decay problems but also clearly represent the dynamics of the fluorophores being investigated. Lastly, a sensorless adaptive optics setup is used for FLIM imaging in deep scattering tissues. The capability of the system is demonstrated in fixed and living animal models, including mice and zebrafish.
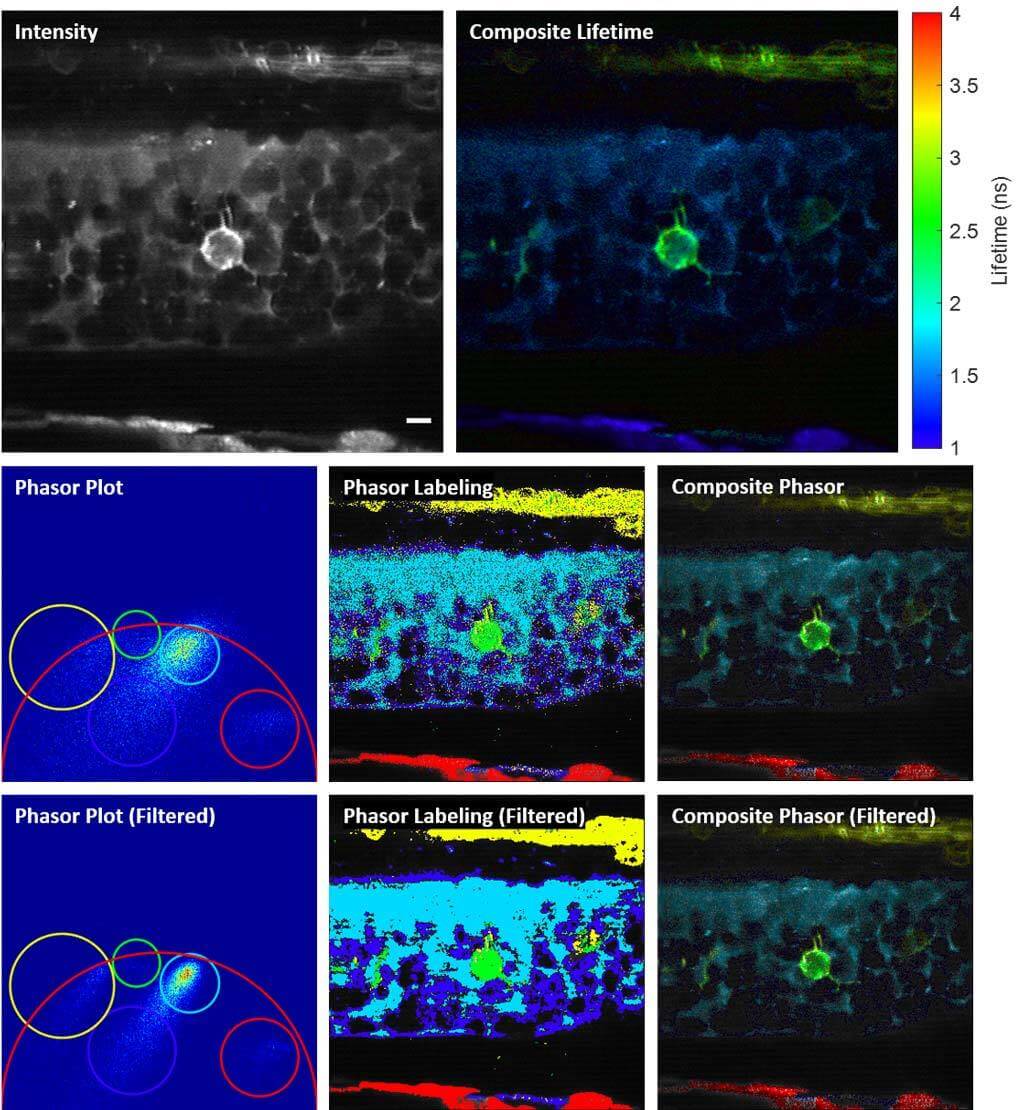
Related Publications:
- Yide Zhang, Ian H. Guldner, Evan L. Nichols, David Benirschke, Cody J. Smith, Siyuan Zhang, and Scott S. Howard, “Three-dimensional deep tissue multiphoton frequency-domain fluorescence lifetime imaging microscopy via phase multiplexing and adaptive optics”, SPIE Photonics West 2019, San Francisco, California USA, Feb. 2019.
Fluorescence Microscopy Denoising (FMD) Dataset and Deep Learning Denoising Methods
Fluorescence microscopy has enabled a dramatic development in modern biology. Due to its inherently weak signal, fluorescence microscopy is not only much noisier than photography, but also presented with Poisson-Gaussian noise where Poisson noise, or shot noise, is the dominating noise source, instead of Gaussian noise that dominates in photography. To get clean fluorescence microscopy images, it is highly desirable to have effective denoising algorithms and datasets that are specifically designed to denoise fluorescence microscopy images. While such algorithms exist, there are no such datasets available. In this paper, we fill this gap by constructing a dataset - the Fluorescence Microscopy Denoising (FMD) dataset - that is dedicated to Poisson-Gaussian denoising. The dataset consists of 12,000 real fluorescence microscopy images obtained with commercial confocal, two-photon, and wide-field microscopes and representative biological samples such as cells, zebrafish, and mouse brain tissues. We use imaging averaging to effectively obtain ground truth images and 60,000 noisy images with different noise levels. We use this dataset to benchmark 10 representative denoising algorithms and find that deep learning methods have the best performance. To our knowledge, this is the first microscopy image dataset for Poisson-Gaussian denoising purposes and it could be an important tool for high-quality, real-time denoising applications in biomedical research.
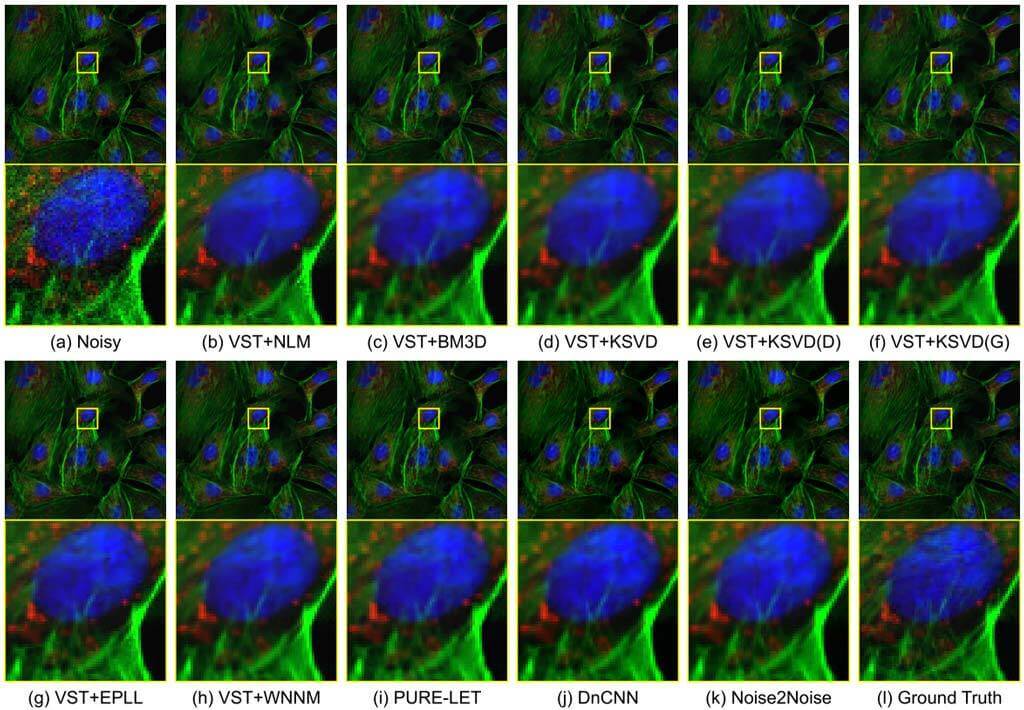
Related Publications:
- Yide Zhang, Yinhao Zhu, Evan Nichols, Qingfei Wang, Siyuan Zhang, Cody Smith, and Scott Howard, “A Poisson-Gaussian denoising dataset with real fluorescence microscopy images”, preprint arXiv: 1812.10366.
Featured Researchers
-

Yide Zhang
PhD Candidate, Department of Electrical Engineering